By Biola Lawal
Abuja (FLOWERBUDNEWS): NAFDAC Boss, Prof. Mojisola Adeyeye has
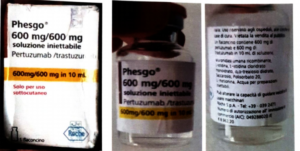
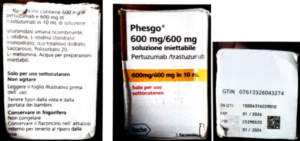
directed a mop up operation across the country to mop up Counterfeit PHESGO 600mg600mg/10ml With Batch Number C5290S20 Stated to be Manufactured by Roche S,P,A.
Prof. Adeyeye directed all NAFDAC Zonal Directors and State Coordinators to ”carry out surveillance and mop up the counterfeit products within the zones and states.”
Flowerbudnews reports that this was announced by NAFDAC in a statement alerting Nigerians of a report of a suspected counterfeit Phesgo® 600mg/600mg/10ml, labeled with batch C5290S20.
Prof. Adeyeye also implored importers, distributors, retailers, healthcare professionals, and caregivers to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the counterfeit products.
She disclosed that Phesgo 600mg/600mg Solution for Injection, ”is used to treat breast cancer. It works by killing the cancer cells and preventing their further growth.”
The NAFDAC Boss stated:
The Marketing Authorization Holder for the original product, (MAH) Roche, received a complaint from a doctor at Lagos University Teaching Hospital (LUTH-NSIA) reporting a suspected counterfeit Phesgo® 600mg/600mg, labeled with batch C5290S20.
The product was reported to have been brought in by a patient for administration. It had not been administered at the time of the report, as it matched the previously reported counterfeit batch C3809C51.
Although no sample was returned to Roche for investigation, only pictures displaying parts of a Phesgo® 600mg/600mg in a 10ml folding box and a labeled vial.
Images of the suspected product were examined by Roche and compared to the genuine samples retained for reference.
The investigation identified the following significant differences between the complaint sample pictures and the genuine materials which confirmed the falsified status of the suspected counterfeit batch of Phesgo® 600mg/600mg.

A non-existent batch number in their database; Associated language does not correspond; Missing Basilisk, incorrect bollino date, and the tamper evidence labels do not correspond to the genuine Roche material.
Chemical analysis was not possible because no physical sample was available for return to Roche.
Phesgo 600mg/600mg Solution for Injection is used to treat breast cancer. It works by killing the cancer cells and preventing their further growth.

Risk Statement:
The illegal marketing of counterfeit medicines poses serious health risks, as it undermines the safety, quality, and effectiveness of these products due to a lack of regulatory compliance provisions.
Product details:
The details of the counterfeit product are as follows: Product Name: Phesgo® 600mg/600mg/10ml injection; Stated Manufacturer: Roche
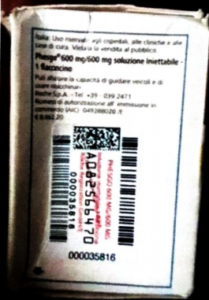
Manufacturing Date: 01/2024
Expiry Date: 01/2026
Batch No: C5290S20
Manufacturing site of the counterfeit product is Roche S, P.A
Note: The correct manufacturing site for genuine Phesgo 600mg/600mg is F.Hoffman La Roche Limited, Wurmisweg, CH-4303, Kaiseraugst, Switzerland.
All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked, She counselled.

Prof. Adeyeye urged healthcare professionals and consumers to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, or call NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
”Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS). (Flowerbudnews)
About Flowerbudnews

Established by Hon. Biola Lawal, a former Acting Managing Director of the News Agency of Nigeria (NAN), FLOWERBUDNEWS is a consortium of active veteran journalists, experienced Multimedia broadcast experts and image makers. We are drawn from both public and private sectors of Nigeria’s media Industry with a common determination to enhance the practice of responsible journalism.
Lawal, on his part, is also a former Honourable Commissioner for Information,Youth, Sports and Culture of Osun state, his home state.
Biola Lawal had also successfully served two tenures as Press Secretary to the ECOMOG Force Commander in Liberia during the Liberian and Sierra Leone Civil wars. He was an outstanding NAN Defence and War Correspondent for many years.
The retired NAN Acting Boss holds the honour of being the only journalist that served two terms on the ECOMOG international assignment due to his high professionalism and decency.
He is a Co-Author of the book; ECOMOG, A BOLD ATTEMPT AT REGIONAL PEACEKEEPING! Edited Mrs Magaret Voght. The book remains the most. factual, detailed and authentic book on the ECOWAS sponsored ECOMOG Military operation.










